VCLS has been selected as one of the experts by BPIFrance to accompany their In Vitro Diagnostics (IVD) start-ups.
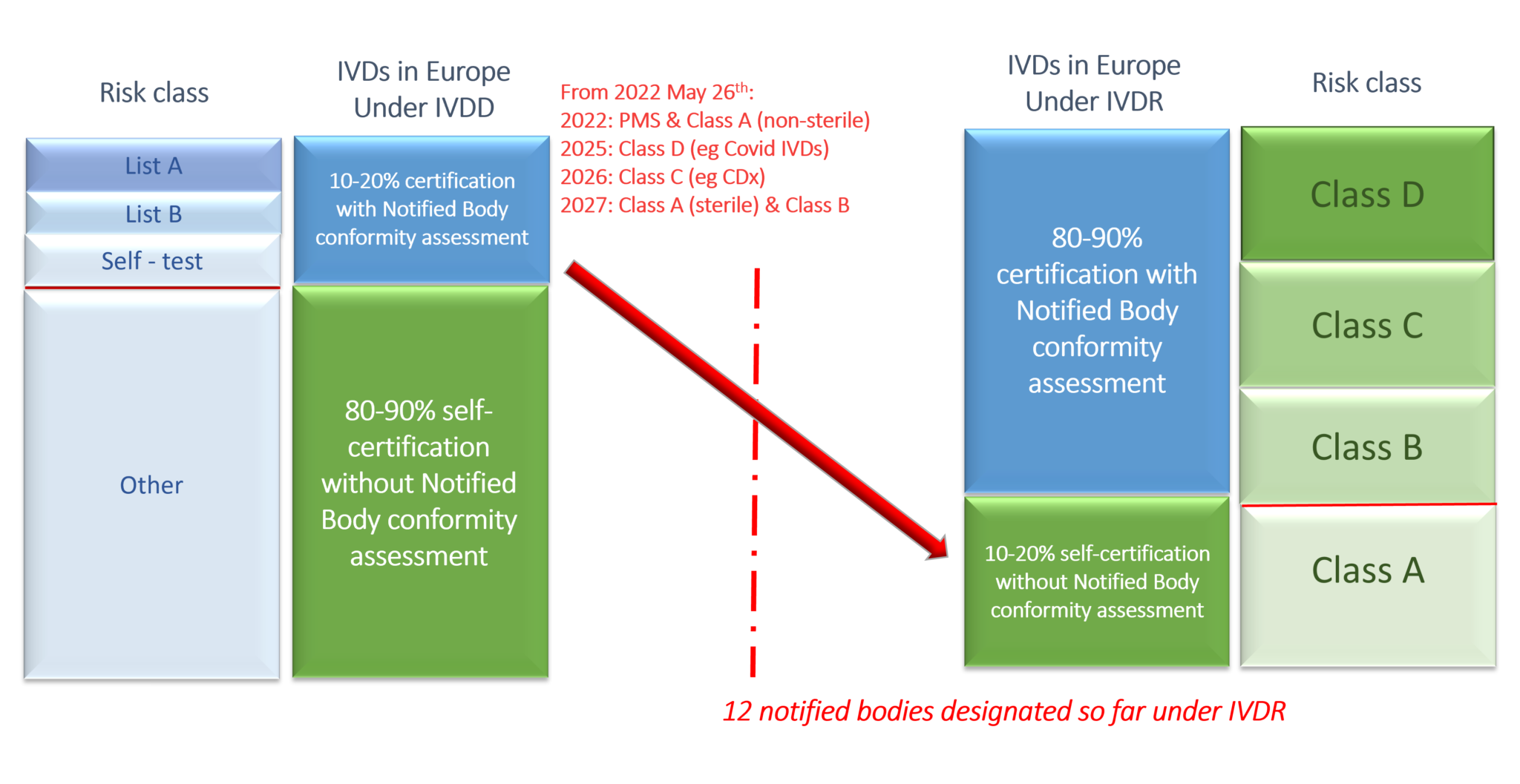
The new EU IVD Regulation (IVDR) entered into force in May 2017 and become applicable on 26 May 2022. The transition from the In Vitro Diagnostic Medical Device Directive (IVDD) 98/79/EC to the new regulation has a great impact on the in vitro diagnostic medical device (IVD) manufacturers. Today, new IVDs will already need to meet stricter requirements to enter the market, whilethe manufacturers of already approved IVDs under the IVDD are allowed an extended transition time ending on 26 May 2025 to 2027 (timeline subject to risk classification of device), to meet the requirements of the IVDR. Making use of the extended transition period, however, means that no significant changes in the design and intended purpose of the device are allowed and vigilance as well as market surveillance requirements under IVDR need to be met.
Here are some key changes:
- Extension of IVD regulation’s scope (genetic tests, companion diagnostics, software)
- Classification based on risk (4 classes: A, B, C and D);
- Involvement of Notified Bodies (NB) for the CE marking of IVD products will increase drastically. As shown in the figure below, only 10 – 20% of the IVDs required a NB conformity assessment under the IVD directive, compared to 90% of the IVDs under the IVDR. The workload of the NBs will therefore rise since only 10 of them are designated under IVDR out of 22 under IVDD, creating a problematic bottle neck for the IVD legal manufacturers and ultimately the patient access to their products.
- General Safety and Performance Requirements of the IVDR are strongly reinforced compared to the Essential Requirements of the IVDD
- Reinforcement of requirements on clinical evidence (scientific validity, analytical performance and clinical performance) resulting in an elaborate performance evaluation report
- Setting out the obligations for the different “economic operators”
- Assignment of a “person responsible for regulatory compliance” (PRRC)
- Identification & traceability with Unique Device Identification (UDI)
- Introduction of central electronic system for vigilance and market surveillance EUDAMED
- Reinforcement of Notified Bodies powers and responsibilities, with possible unannounced site inspections and sample checks
- Assessment of high-risk products by the European Medical Device Coordination Group (MDCG)
- Role of EU Reference Laboratories (specific hazards and technology assessment) for Class D IVDs
An “in vitro diagnostic medical device” (IVD) is a medical device, reagent, instrument or software, which is intended to be used in vitro for the examination of specimens derived from the human body, for diagnostic purposes. These diagnostic tests may provide information concerning a pathological disease or a predisposition to a medical condition or disease, but may also predict treatment response or reactions (companion diagnostic – CDx).
IVD tests may be performed in clinical laboratories, while devices for “near-patient testing” are intended to be carried out by healthcare professionals outside of a laboratory environment. Self-testing devices are another category of IVDs, intended to be used by lay persons.
With healthcare systems driving for greater efficiency and sustainability, IVD tests are providing information necessary to increasingly focus on prevention, early intervention and disease treatment. Consisting of diagnostic tests performed at the hospital or private clinical laboratories, near-patient testing and self-tests, the IVD market is expected to continue to grow at a steady pace within a substantially more stringent international regulatory environment.
Key challenges encompass:
- Product qualification/classification
- Market clearance route
- Safety and Efficacy claims
- Risks analysis and risk management plan
- Performance evaluation (including clinical utility)
- Quality Management System (QMS)
- Market access strategy and value demonstration to payers
VCLS Solutions
Science-based, global product development strategy and planning
- Support the development of appropriate product positioning and claims
- Assist in innovative product qualification/classification and regulatory development plans
- Develop roadmaps towards international market approvals (EU, UK, US, Australasia, South America)
- Advise on/conduct regulatory bodies strategic and product development meetings (Notified Bodies, EU Competent Authorities, Food and Drug Administration)
- Support with selection of EU Notified Body and UK Approved Body
Tactical operations
- Assist with risk analysis and risk management plan, including fulfilment of requirements for usability
- Develop and submit regulatory files (EU CE and UKCA mark Technical File, US 510(k), PMA and De Novo, Canada, Australasia, South America)
- Optimize quality systems, including vigilance
- Advise on labeling and advertising



