
Have you ever asked yourself how, as a patient, you would react if your physician proposed you to participate in a clinical trial? Would you accept, and what would be your reasons to accept or refuse?
In this post, we will see why understanding the perspectives and preferences of patients and his representative or caregiver is essential while developing a clinical trial.
It’s unmistakable that clinical trials are crucial for healthcare industries and without successful trial, a new product cannot be brought to market.
Recruitment and retention in clinical trials are huge challenges and constitute a real and costly threat.
Poor recruitment is among the most frequent and important reasons why a study is prematurely discontinued. Indeed, 85 % of clinical trials fail to recruit enough patients [1] and 80% are delayed or closed because of problem of recruitment [2].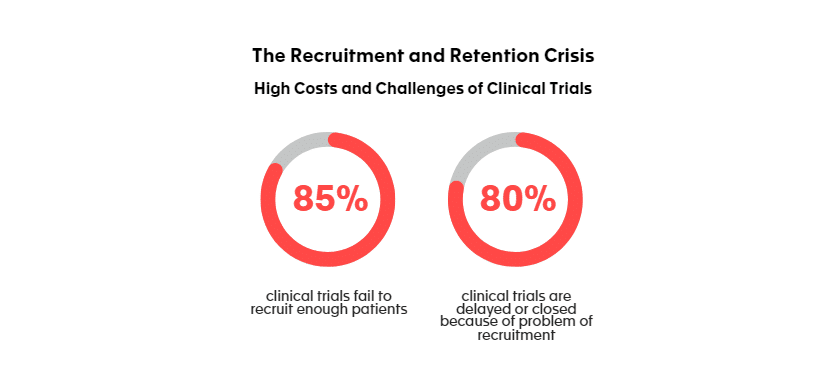
And when we know that the estimated cost of patient recruitment is 40% of the total pharma research budget, or $1.89 billion [3] and that delays can cost sponsors between $600,000 and $8 million for each day that a trial delays a product’s development and launch [4], there is no doubt on the importance to be brought on the recruitment strategy.
Among the most frequently reported reasons for recruitment failure in Randomized Controlled Trials RCTs [5] are:
- overoptimistic recruitment estimations
- too narrow eligibility criteria
- lack of engagement of recruiters/trial team
- lack of competence/training/experience of recruiters
- insufficient initial funding and finally
- the high burden of trial’s participants.
And indeed, there is an actor that is essential in the building of a strong and efficient clinical trial ‘s strategy: the patient. It’s fair to say that as final users of a solution, patients must have a greater influence on the decisions that affect them and should actively participating, among other important steps from the product’s development, in the design and conduct of clinical trial.
How can a patient de–risk a clinical trial?
There are many reasons that can lead to a trial failure. There might be a way to decrease some of these risks by collaboratively working with patients.
It has been showed that 30% of trials are cancelled due to insufficient participation, that 30% of patients drop-out before the study ends, and finally that 30% do not sign the inform consent due to a lack of understanding [6,7,8]. It also appears that 23% of patients are unhappy with study location. All these barriers will result in severe consequences for the sponsor (but also for the patients). Indeed, losing a patient also means a loss of valuable data, failure to retain patients can cause costly delays in trials and lengthy trials may also delay patient’s access to potential beneficial and innovative treatments.
It’s essential for the sponsor to gather as early as possible information about patients’ preferences for the way a trial is organized as this could enhance the participation rate. Moreover, there is a need to validate that trial design and implementation match participant abilities and circumstances, identifying elements of burden that may become risks to participation but also retention in clinical trials.
What are the factors contributing to the difficulties of recruitment and retention from a patient perspective?
- The study design itself. By collaborating with patients, sponsor can avoid incorrect assumptions regarding patient’s preferences, unmet needs and desired outcomes. Sponsor must verify if selected endpoints also reflect the patient’s reality, whether eligibility criteria are realistic or not. Are the patient-reported measures captured in a practical way (i.e. on paper, tablet, etc.)?
- It’s important for the sponsor to measure the importance of the trial’s burden for the participants and /or their caregivers. Travel to study sites can cause fatigue and can involve a need of day off. Long duration or too high frequency of study visits as well as heavy medical/follow-up procedures can also raise huge concerns for patients. Do not also underestimate the burden linked to frequent and lengthy questionnaires. It could also be interesting for the sponsor to understand if alternatives to study visits (nurse home visits or telemedicine) would improve patient participation? These questions and considerations, so relevant to clinical trial participants and sponsors can be answered by engaging and collaborating with patients.
- Other important aspects to be considered for “de–risking clinical trials” by engaging with patients are related to recruitment strategies and inadequate communication. There is an urgent need to improve access to clinical trials and it’s always striking to see for example that participation in clinical trials typically does not exceed 5% of patients with cancer [9]. This is because only patients whose doctors are also investigators participate in clinical trials.
By working collaboratively with patient organizations and communicate efficiently and regularly, Patient Organizations should receive adequate, accurate information, so they can pass it along with their associates and increase the awareness and visibility of a specific trial.
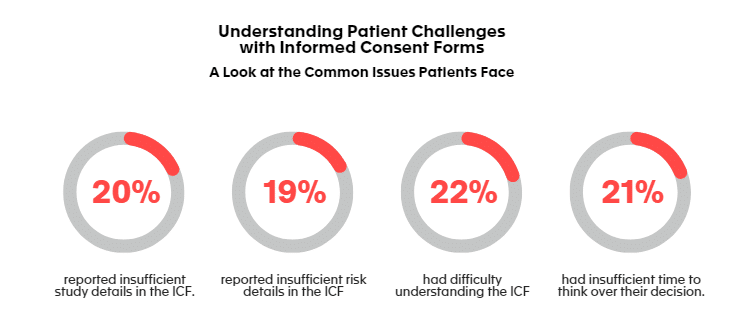
To avoid situation where patients do not sign the ICF (Inform Consent Form), as mentioned earlier in this post, it’s also essential for them to receive all the information they need to make a free and informed choice before consenting to participate in a study. A study performed some years ago [10] among the patients revealed that:
- 20% of patients said that the ICF did not contain enough details about the study itself or about the risks taken while participating.
- 22% of patients said that it had been difficult for them to understand the content of the ICF.
- 21% of patient said that they had not had enough time to think over their decision before signing the ICF.
To ensure an ICF has suitable information, written in a legible and plain language, who is the best placed? Again, it’s the patient who could play an important role in the review and evaluation of documents such as the ICF, as well as all patient–facing material. It’s essential that these educational materials are culturally appropriate and tailored to multiple educational levels.
Conclusions
Overcoming clinical trial recruitment and retention challenges is key in the success of a clinical trial and it’s still a persistent threat for the sponsor. It exists different ways to reduce this risk and one of them is starting by adopting a strong patient-centric approach, where the patient is considered as a real expert and partner in the clinical trial process. By working collaboratively with patients, caregivers and patient organizations, listening their voice and taking into account their insights, understanding and evaluating their needs and preferences, planning and execution of a clinical trial might be hugely optimized.
Never forget that clinical trial participants are real people and volunteers, and their experiences matter in shaping better healthcare practices.
References:
[1] Idnay, B., Butler, A., Fang, Y., Li, Z., Lee, J., Ta, C., Liu, C., Ruotolo, B., Yuan, C., Chen, H., Hripcsak, G., Larson, E., & Weng, C. (2023). Principal Investigators’ Perceptions on Factors Associated with Successful Recruitment in Clinical Trials. AMIA Joint Summits on Translational Science proceedings. AMIA Joint Summits on Translational Science, 2023, 281–290.
[2] Decentralized clinical trials: Are we ready to make the leap? | BioPharma Dive
[3] Considerations For Improving Patient Recruitment Into Clinical Trials (clinicalleader.com)
[4] Lawrence, C. E., Bruce, V. N. M., Salberg, L. D., Edwards, T., Morales, C., Palm, M., & Bernard, G. R. (2023). Quantitative assessment of the impact of standard agreement templates on multisite clinical trial start up time. Journal of clinical and translational science, 7(1), e204. https://doi.org/10.1017/cts.2023.622
[5] Briel, M., Elger, B. S., McLennan, S., Schandelmaier, S., von Elm, E., & Satalkar, P. (2021). Exploring reasons for recruitment failure in clinical trials: a qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada. Trials, 22(1), 844. https://doi.org/10.1186/s13063-021-05818-0
[6] 2015 Why Clinical Trials Are Terminated
[7] National Academy of Sciences. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, D.C.: National Academies Press; 2010. Available at: www.nap.org
[9] The Patient Access to Cancer Care Excellence (PACE) [homepage on the Internet]. Available from: https://pacenetwork.com/. Accessed September 28, 2015
[10] Sacristan JA, Aguaron A, Avendaño C, Garrido P, Carrion J, Gutierrez A, Kroes R, Flores A. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence. 2016;10:631-640. https://doi.org/10.2147/PPA.S104259
Published on: Jun 17, 2024






