
Raw and starting materials are an essential part of cell-based and gene therapy products (CGT). Frequently, the development of Cell and Gene Therapy (CGT) products starts in an academic environment, and the raw and starting materials may be selected without considering if they will be suitable in terms of quality for a clinical trial submission, and later, for a marketing authorization. Consequently, the lack of an appropriate level of quality can create complications as the product progresses further into clinical development. The transfer of manufacturing from an academic to a cGMP facility for clinical trial product manufacture involves potential changes in starting material supplier and quality with the need to switch from Research Grade to Clinical Grade material.
Quality requirements for the materials used in the process strictly depend on their contribution to the final product. Often, we observe a lack of clarity defining the quality of materials that have been used to manufacture early product batches and a need to properly establish material quality testing to ensure it is suitable for use in the manufacturing of clinical material.
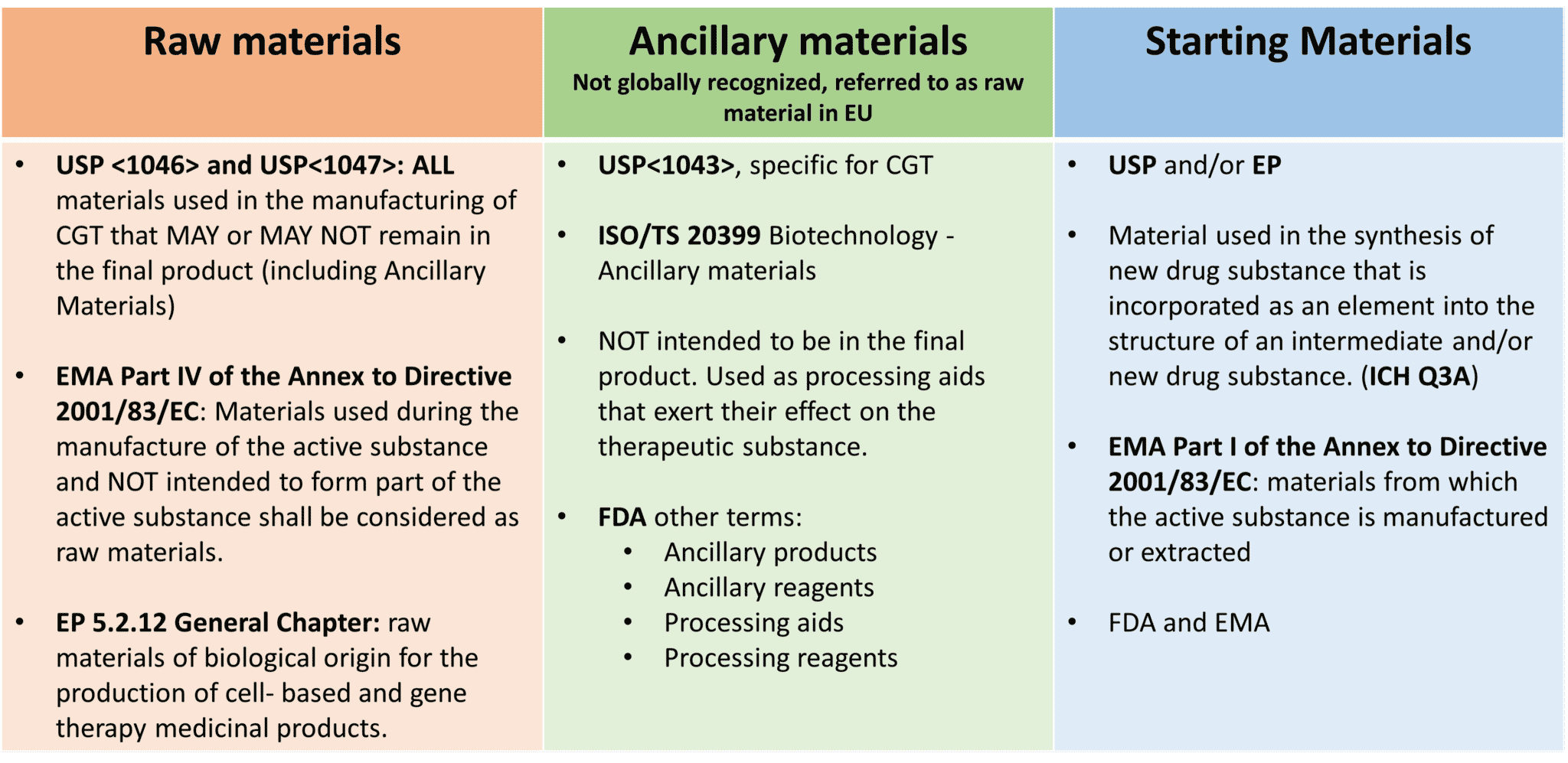
The terms applied to materials used in manufacturing of CGT products are not as clear compared to their use in small molecules and even for more traditional biological products. The terminology in the context of cell and gene therapy is quite complex. The complexity in defining if a material belongs to one classification or another is due to the fact that the same material can act differently depending on how it is used, and what the function is for each therapeutic product. In addition, definitions are not fully harmonized globally, contributing to the complexity. The definitions below aim to provide some clarity to the terms used in the United States and Europe.
Definitions
USA
- Raw Materials are defined in United States Pharmacopeia (USP) <1047> and USP<1046> as ALL materials used in the manufacturing process and may include cells, tissues, biological fluids, growth factors, and natural and synthetic biomaterials. From the USP, Raw Materials may or may not remain in the final product.
- Ancillary Materials are defined in the USP <1043> and are a subset of Raw Materials that come into contact with the cell or tissue product but are NOT intended to be part of the final product. These materials can be processing aids that exert their effect on the therapeutic substance. This term is also discussed in FDA documents and guidance and is also referred as ancillary products, ancillary reagents, processing aids, and process reagents.
Europe
- Raw Materials are defined in the European Pharmacopeia 5.2.12 as materials used during the manufacture of the active substance and NOT intended to form part of the active substance.
- The term Ancillary Materials is not used.
USA and Europe
- In both regions, the term Starting Materials is defined as materials forming an integral part of the active substance. And by that definition, every starting material is a critical material.
A summary of the terminology is provided in Figure 1 below.
Impact of the definitions
Understanding these definitions and properly classifying the materials used in the manufacturing process of the CGT product will allow for the establishment of the adequate testing and control of these materials. It is important to remember that release testing of the final product will only confirm the quality of the product and will not modify the quality. Product quality is defined by the manufacturing process itself: how well it controls quality and how much variation it allows. Therefore, the quality of the materials (raw, starting, and ancillary) is fundamental for the final quality of the CGT product.
Stay tuned for Part 2 of this blog where we will cover the implications of the Starting Materials in the Regulatory Dossier.
Additional reading:
- USP<1046>
- USP<1043>
- EP 5.2.12
Published on: August 30th






