From pre-market product safety to post-market surveillance, we provide integrated vigilance services all along your product life cycle.
Safety of a product is assessed all along its lifecycle , from early development , clinical trials , marketing authorization to post-marketing. Our global safety and vigilance team collaborates with clinical operations, regulatory, and others, to manage your safety and vigilance needs at any stage of product development to ensure quality and compliance.
A set of pharmacovigilance services to support you throughout the entire product lifecycle
- Safety needs analysis and system design
- PV Quality Management System
- Safety database set up, maintenance and MedDRA updates
- Post-market material set up and maintenance
- Clinical trial material customization and maintenance
- Agency Admin set up, maintenance EudraVigilance profile / XEVMPD updates / MHRA profiles / FAERS / VAERS
- Adverse event collection, processing, analysis, reporting and follow up
- Aggreagte reports: DSUR/ASR, PSUR/PBRER/PADER
- QPPV office (SDEA, Partners management, EU/UK/Local QPPV appointement, PSMF, KPI…)
- International and local literature surveillance
- RMP / REMs
- Signal management
- Medical information
A vigilance system that grows with your needs
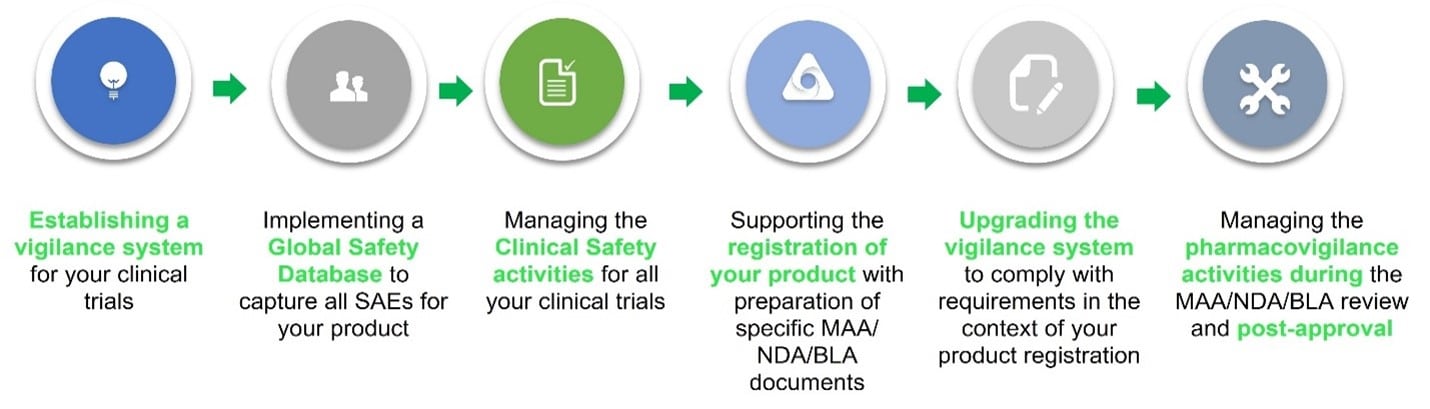
Global pharmacovigilance resources with extensive experiences
- Ability to work around the clock via a global vigilance team
- Team members with average of 10 years’ experience
- Agile and dedicated project management
Years in the vigilance market

Non-stop working window

Locations in EU and Asia
A range of pharmacovigilance tools to satisfy your needs
At VCLS, we employ an intuitive, unified, cloud-based safety system trusted by industry leaders to help you boost efficiency, simply process and streamline operations.
Readily accessible
- SaaS / Web-based access
- Secured user identification
Secured hostage
- AWS and Back-up sites in Europe
- Tier III+ / ISO 27001 / HADS
Regulatory reporting
- E2B compliant (R3,compatible R2)
- XML files & gateways option
- CIOMS I / MedWatch forms
- Line listings and Summary tabulations
Specific features
- Automation
- Artificial Intelligence



