Late-stage Clinical Development Solutions
Transform your promising therapies into successful market-ready treatments with our expert late-stage clinical services
From seamless Phase 2 and Phase 3 trials to strategic regulatory navigation, our dedicated team ensures precision, compliance, and speed every step of the way.

SOLUTIONS
What we can help you achieve:
We provide tailored solutions of clinical research through a collaborative and partnership-driven approach

- CMC and regulatory strategy for marketing registration (MAA, BLA, NDA)
- CMC compliance, support for CRO/CDMO selection and management
- Authoring Module 3 of MAA, BLA, NDA

- Health Economics and Outcomes Research (HEOR) including value proposition development and cost-effectiveness analysis
- Early access program design and execution
- Payer and HTA interaction & engagement

- Pharmacovigilance (PV), including safety signal detection, risk management planning, Adverse Event reporting, and Periodic Safety Updates Reports (PSURs)/Development Safety Update Reports (DSURs)
- Post-Market Surveillance (PMS) & device vigilance services

- Guidance on regulatory requirements and compliance for global markets (FDA, EMA, etc.).
- Preparation and submission of INDs, NDAs, BLAs, MAAs, and CTAs.
- Interaction with regulatory agencies

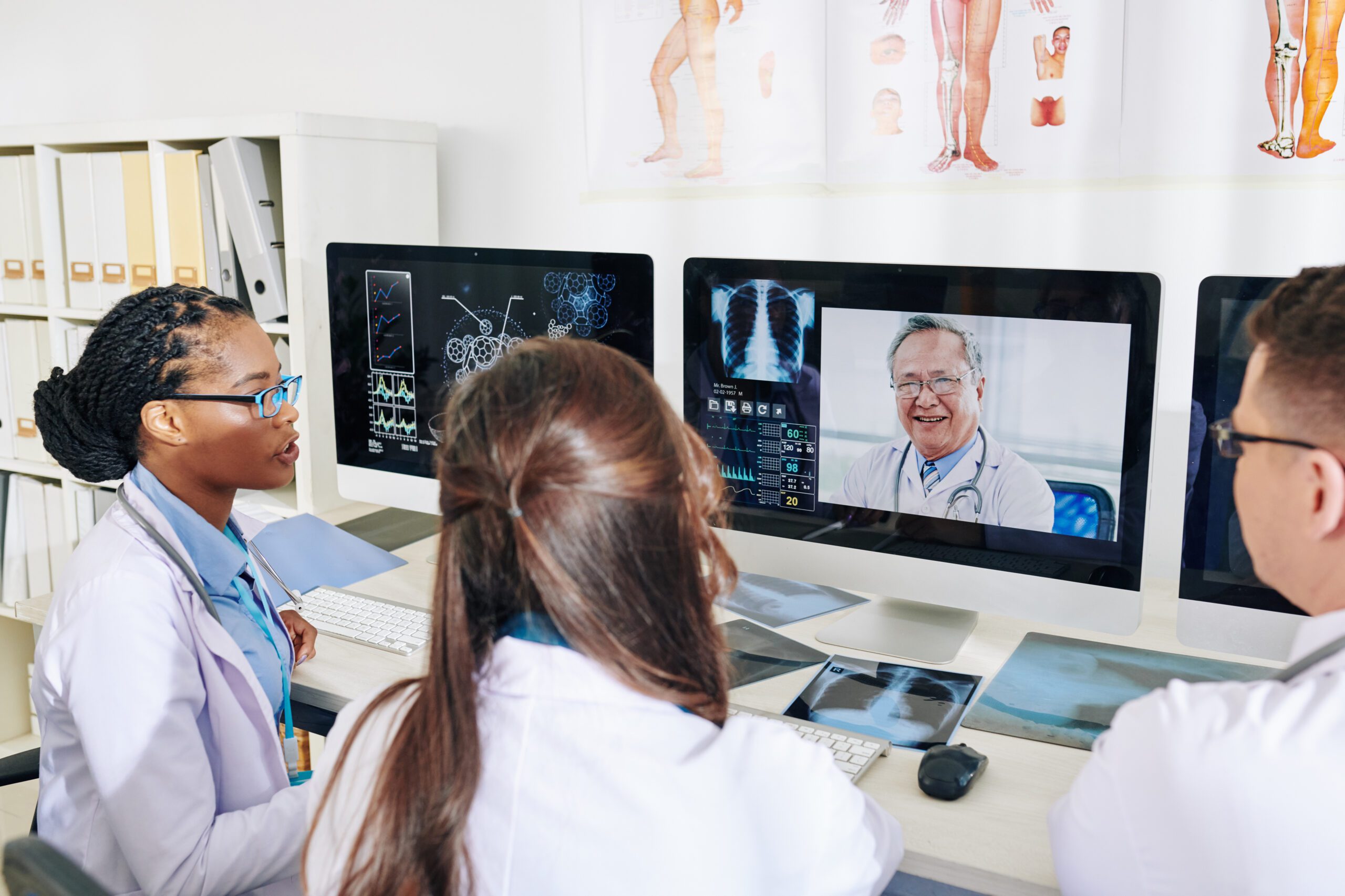
Comprehensive End-to-End Clinical Development Services for Seamless Market Entry
Our end-to-end services encompass everything from regulatory strategy to market access, providing a seamless and integrated approach that streamlines your path to market approval.
Proven Track Record of Success
We have a history of successfully guiding biotech companies through regulatory hurdles and market entry challenges. Our experience spans multiple therapeutic areas and regulatory frameworks, ensuring that your project is handled by experts who understand the biotech landscape from every angle.

FAQs
How many patients do we need to recruit in Phase 3 trials?
The number of patients needed in a Phase 3 clinical trial varies significantly based on factors like the primary endpoint, disease type, expected treatment effect size, statistical power, and regulatory requirements.
The main considerations for determining sample size are:
- Statistical power and significance level
- Effect size, including expected treatment effect and minimally clinically important difference
- Primary endpoint
- Control group and randomization ratio
- Dropout rate
- Regulatory guidance and disease specifics
Finally, consulting with a biostatistician early in trial design is essential to determine an accurate sample size that balances scientific rigor with practical feasibility.
read more
What are the main endpoints for Phase 3 trials?
The main endpoints of Phase 3 trials are chosen to demonstrate the efficacy, safety, and clinical benefit of a treatment, as they serve as the basis for regulatory approval and subsequent clinical use.
The primary endpoint is typically the key measure of the treatment’s effectiveness. Examples include overall survival (OS), progression-free survival (PFS), event-free survival (EFS), symptom relief or improvement, cure rate or remission rate.
Regulatory agencies like the FDA and EMA provide guidance on acceptable endpoints for specific indications. Aligning endpoints with regulatory expectations is crucial. Endpoints that are meaningful to patients – including survival, symptom relief, and improved functionality – enhance the likelihood of the treatment’s acceptance and real-world use. Endpoints should also reflect outcomes that significantly impact patient health and quality of life, ensuring that trial results translate into real-world clinical benefits.
Therefore, Phase 3 trial endpoints are thus carefully selected to balance scientific rigor, regulatory requirements, and patient relevance, ensuring that the data generated will support regulatory approval and demonstrate meaningful benefits to patients.
read more
Related Glossary

Questions? Get the answers from our expert team
No two product development paths are the same. Talk to our experts about your development challenges and we will provide you actionable recommendations.