SOLUTIONS
Each development plan is unique. We tailor our solutions to your needs and situation.
Worldwide expertise for a seamless entry into global markets
Whether you are targeting the US, Europe, China or other global markets, our end-to-end services ensure your product meets all regulatory and market access requirements to achieve the most optimized market entry, faster.
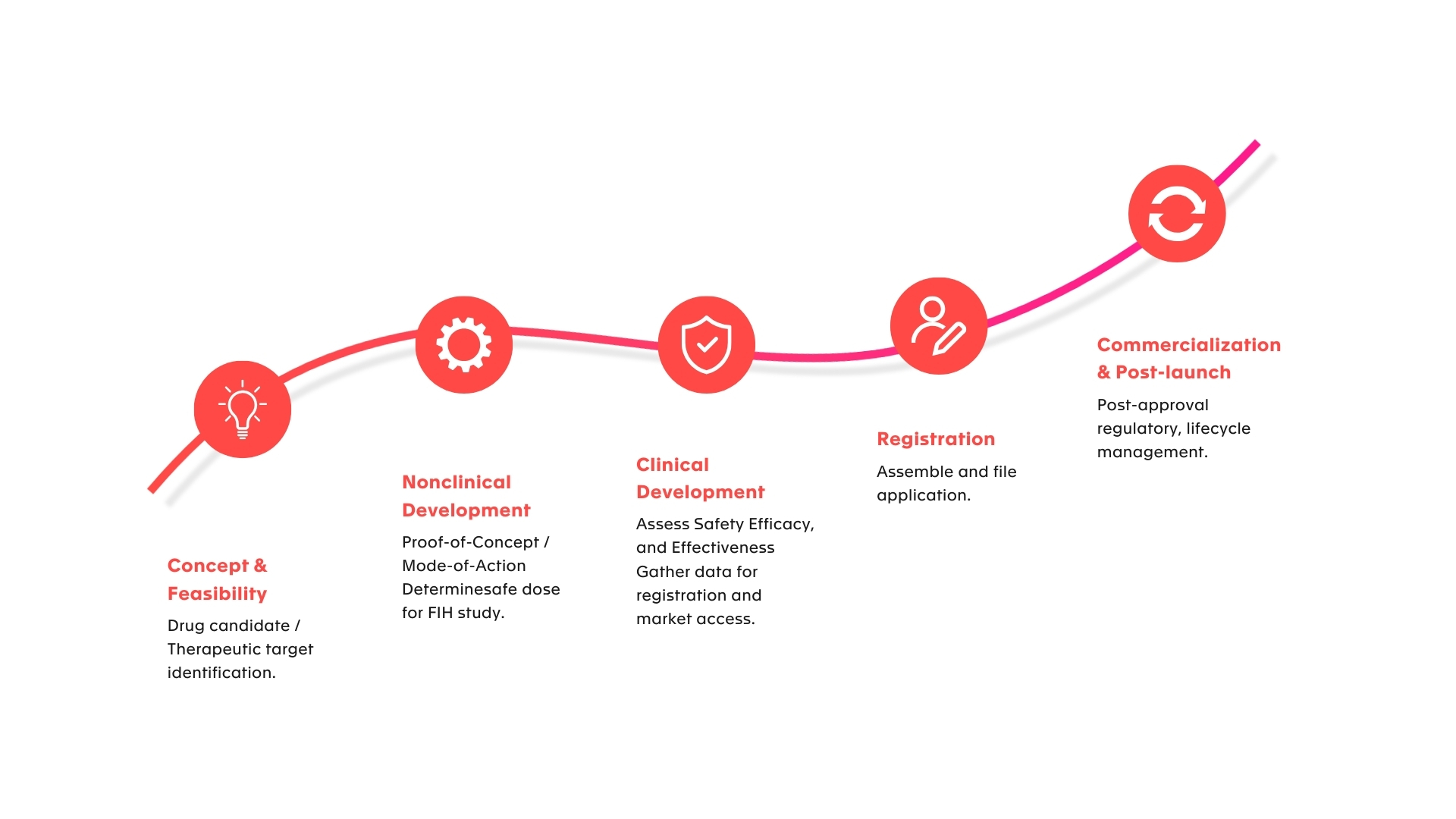
Patient-centric solutions throughout your development journey
A customized “one-stop shop” from preclinical to product launch, and beyond. We are your dedicated and trusted partner, empowering you every step of the way.
Flexible solutions adapted to your development needs through operational excellence
Whether you’re an emerging biotech needing both expertise and resources, or an established pharmaceutical company seeking additional support, we provide tailored solutions that align with your goals. We work closely with you to build a strong partnership, driving operational success together.

Enriching your HealthTech development journey to success with our integrated solutions
Whether your health technology is in biologics, medical devices, digital solutions, or diagnostics, we provide you game-changing solutions tailored to your technologies.

VCLS marks new era as global HealthTech strategic partner with integrated solutions for the TechBio age

Why partner with VCLS?
-
Holistic services & integrated product development
-
Bridging scientific innovation and commercial success
-
Translation science into patient access by leveraging the latest technologies

THEY TRUST US
Join the ranks of satisfied clients who trust our reputable services. Our history of positive testimonials and industry recognition speak volumes about our commitment to excellence and client satisfaction.
Careers
Work at VCLS

In both CMC and choral singing, it’s all about listening, precision, and harmony — individually and as a team.
Nicole Cohen, PhD
Senior Director, CMC at VCLS

Questions? Get the answers from our expert team
No two product development paths are the same. Talk to our experts about your development challenges and we will provide you actionable recommendations.