FDA Meeting Series: How, When and What – INTERACT Meetings

Published on: Sept 28, 2023
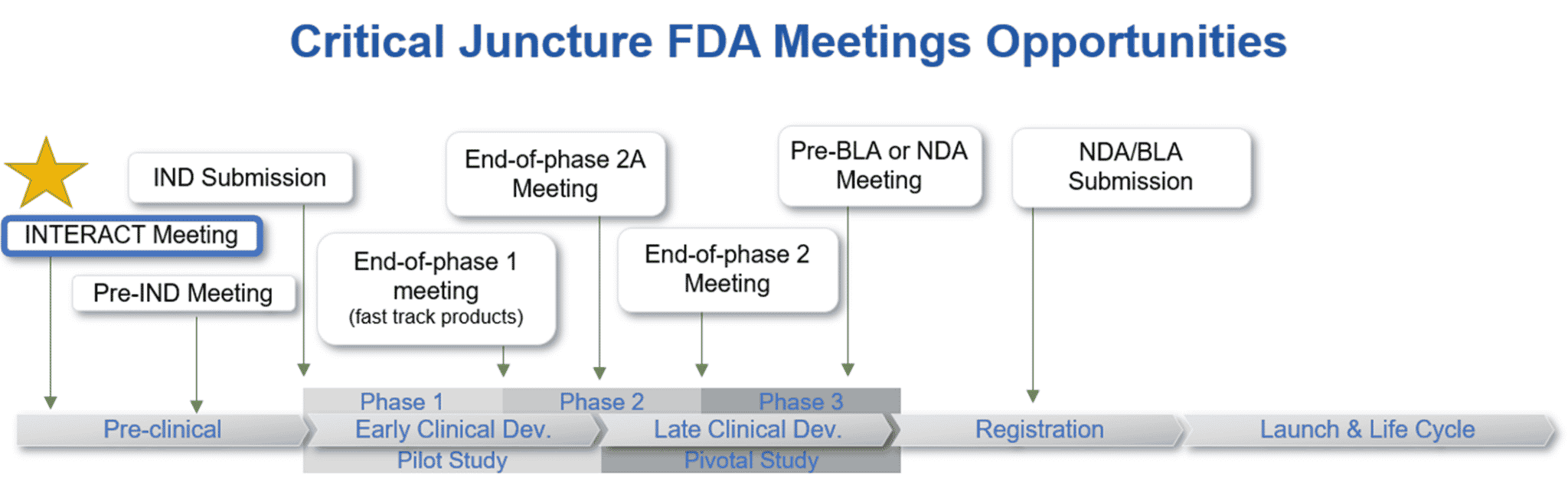
INTERACT Meetings
What:
CDER is now also accepting INTERACT Meetings. Initial Targeted Engagement for Regulatory Advice on CBER/CDER Products (INTERACT) Meeting are informal meetings held between sponsors of innovative investigational biological products and the Center for Biological Evaluation and Research (CBER), or the Center for Drug evaluation and Research (CDER). The issues typically relate to IND requirements, for example, questions about design of IND-enabling toxicity studies, complex manufacturing technologies or processes, development of innovative devices used with a drug or biologic, or the use of New Approach Methodologies. INTERACT meetings are intended to facilitate IND-enabling efforts when the sponsor is facing a novel, challenging issue that might otherwise delay progress of the product toward entry into the clinic in the absence of this early FDA input. The sponsor needs to have selected a specific investigational product or a product-derivation strategy to evaluate in a 182 clinical study before requesting an INTERACT meeting. CBER and CDER recognize that the development of innovative investigational products can introduce unique challenges related to unknown safety profiles, complex manufacturing processes, technologies and issues, incorporation of innovative devices and the use of cutting-edge testing methodologies that can benefit from early agency input.
INTERACT meetings can:
-
Assist sponsors conducting early product characterization and preclinical proof-of concept studies
-
Initiate discussions for novel devices
-
Inform sponsors about overall early-phase clinical trial design elements
-
Identify critical issues or deficiencies for sponsors to address during development
When:
INTERACT meetings are for products that have begun the development process but have not yet reached the stage where a pre-IND meeting would be appropriate. Before requesting an INTERACT meeting, a sponsor should have selected a specific investigational product or a biological product-derivation strategy to evaluate in a clinical study.
How:
Prior to the first meeting planned with FDA a PIND number must be requested. The PIND number should be requested from CBER by email (CBERRIB@fda.hhs.gov) 30 days prior to the planned meeting request submission date.
Additionally, before requesting an INTERACT meeting a meeting package of no more than 50 pages should be prepared that includes the following information:
-
A description of the product and the disease or condition being treated or prevented
-
A summary of information about product development to date and any future development plans, if appropriate
-
A list of questions with a summary for each explaining the need or context. Questions should be grouped by the following topics (Note: questions regarding combination products should be grouped together):
-
Chemistry, Manufacturing, and Controls (CMC)
-
Pharmacology/Toxicology
-
Clinical
-
A summary of the data to support discussion organized by topic and question
-
A list of all participants, with their titles and affiliations, who will be in attendance from the sponsor’s organization including consultants and interpreters
-
Optionally, the sponsor may include suggested dates and times for the meeting including non-availabilit
INTERACT meeting requests should be made by email (cberdcc_emailsub@fda.hhs.gov, with OTPRPMS@fda.hhs.gov in cc line for Regulatory Management Staff awareness) and should include a cover letter and the meeting package with the request. The cover letter and email subject line should clearly identify the purpose as a request for an INTERACT meeting and the CBER Office where the request is directed. In the meeting request sponsors should define specific areas of input requested from CBER.
If an INTERACT meeting is granted, they will generally be scheduled within 21 calendar days of receipt. The meeting will be held via teleconference within 75 calendar days of the request receipt and will usually be 1 hour in duration. FDA’s preliminary responses will be sent no later than 5 days before the meeting to facilitate discussion. . The requestors responses will be given no later than 3 days from the receipt of Preliminary Responses. No additional questions will be accepted. If the sponsor finds the written responses provided by CBER sufficient and does not desire further discussion, they may cancel the meeting.
During the INTERACT meeting, discussions will focus on and be limited to the initial questions submitted in the meeting package. CBER advice given during INTERACT meetings is informal and non-binding and therefore official meeting minutes will not be issued to the sponsor. Additionally, any meeting minutes prepared and sent to CBER by the sponsor will not be reviewed or evaluated for accuracy. Preliminary responses will be resent within 30 calendar days if the advice provided changes because of the meeting.
If a meeting request is denied the sponsor will be given a reason for the denial. Examples of reasons for denial include:
-
A meeting package dose not accompany the INTERACT meeting request.
-
The meeting package is substantially deficient, significantly limiting the ability to provide constructive feedback.
-
The requested feedback is outside the scope of an INTERACT meeting.
-
The stage of development is either premature or too advanced for an INTERACT meeting. CBER will generally inform the sponsor if a different meeting type is more appropriate.
-
A previous meeting for the same purpose had already been held and no substantially new information has become available.
-
The requested feedback is not appropriate for a meeting with CBER.

Questions? Get the answer by our expert team
No two product developments are the same, talk to our experts about your development challenges and we will provide your actional recommendations.