What Defines a Healthy Microbiome?
Published on: Jul 7th, 2025
Introduction
A “healthy” microbiome plays a key role in maintaining human health by supporting digestion, skin balance, reproductive function, immune response, and overall physiological balance. Yet, defining what exactly constitutes a healthy microbiome remains a major scientific challenge due to individual variability, environmental influences, and methodological inconsistencies.
This article explores the definition, function, biomarkers, and current research technologies around the gut microbiome, with a focus on its implications for diagnostics, therapeutics, and personalized medicine.
Overview of Human Microbiomes
It’s important to remember that the human body hosts several microbiomes, each with its own role in maintaining health and homeostasis.
The main places where microbiome reside are:
- Gastrointestinal tract: the most studied microbiome, comprising ~95% of the body’s microbes.
- Oral cavity: where microbiome is involved in digestion and systemic inflammation.
- Skin: where it contributes to immune defense and barrier protection.
- Vagina: vaginal microbial residents play a key role in reproductive and urinary health.
- Respiratory tract: where microbiome components are involved in respiratory immunity.
The microbiome in the different parts of the body interacts with its host and with other microbiome, forming a complex, dynamic ecological network, that evolves over time and in response to both internal and external factors.
Defining Health and the Role of the Microbiome
Since the original WHO definition of health (1948)* as a “state of complete physical, mental and social well-being”, modern interpretations have evolved to reflect the dynamic and multifactorial nature of health. Health is now seen as an adaptive balance across physical, psychological, social, and existential dimensions.
In this context, human microbiome – is a collection of microorganisms living in and on our bodies- has emerged as a key player in health. The microbiome continuously switches in response to host and external factors such as age, nutrition, lifestyle, or disease, forming a unique bionetwork for each individual (see figure 2).
Humans are not isolated biological systems. We live as holobionts, i.e. integrated assemblages of host and microbial life. Our health depends on the dynamic interactions between our own cells and trillions of microbial partners. These relationships can be commensal, symbiotic, or pathogenic, forming a living ecosystem where balance is key to maintaining well-being.
What Is a “Healthy” Microbiome?
Despite decades of research, there is still no universal definition of what constitutes a “healthy microbiome”. Microbial diversity, resilience, and function are often considered as markers, but many variables, such as geography, ethnicity, diet, and methodological approaches, can significantly influence microbiome composition and therefore complexify the interpretation of findings across different studies.
Key challenges include:
- High inter-individual variability
- Lack of methodological standardization
- Underrepresentation of minority populations
- Difficulty in capturing the microbiome’s dynamic behavior
What Factors Influence the Human Microbiome?
Several core factors determine the composition and function of the human microbiota. Understanding these is critical when defining “health” in a microbial context:
- Age: Microbial diversity and function shift across the lifespan.
- Diet and nutrition: Macronutrient balance, fiber intake, and fermented foods shape microbial populations.
- Drug use: Antibiotics, proton-pump inhibitors, and others affect microbial abundance.
- Lifestyle: Sleep, stress, exercise, and smoking can all modulate the microbiota.
- Environment: Geography, pollution, and urban vs. rural living influence exposure.
- Host genetics: Genetic factors shape immune response and microbial tolerance.
- Hormonal status: Puberty, pregnancy, and menopause impact microbial balance.
- Disease or immune dysfunction: Chronic illness alters microbial resilience and function.
Focus on the Gut: What Is a Healthy Gut?
Lately, the interest for the industry is focused mainly on the gut, skin and vaginal microbiome. Here let’s focus on the gut.
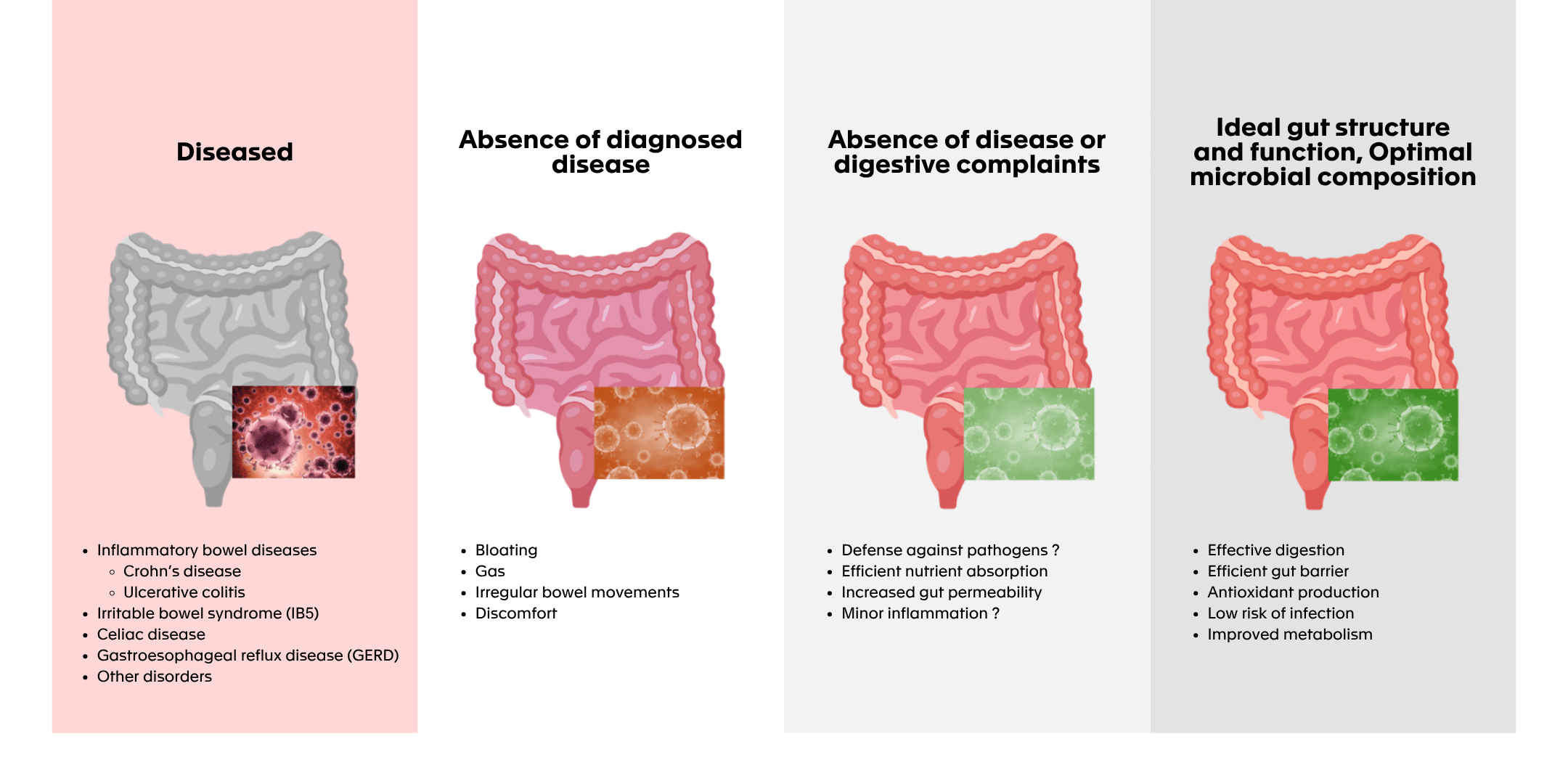
From a functional or clinical viewpoint, a “healthy gut” is considered as not having any diagnosed digestive diseases or disorders which focuses on the lack of detectable medical condition affecting GI tract (e.g., inflammatory bowel disease (IBD) like Crohn diseases and ulcerative colitis (UC), irritable bowel syndrome (IBS), coeliac disease, gastroesophageal reflux disease (GERD) or other functional or structural disorders).
The following figure illustrates the different ways in which gut health can be assessed from clinical diagnosis to symptom perception, to functional performance of the microbiota. It visually highlights how the concept of a “healthy gut” is not binary but context-dependent.
Figure 1: “Healthy” gut, where to put the line? (modified from (Van Hul et al., 2024)**)
How Microbiome Supports Gut Health
A healthy gut microbiome is notably characterized by:
- High bacterial diversity
- Production of key metabolites (short-chain fatty acids, bile acids, tryptophan derivatives)
- Strong immune modulation
- Resilience to disturbance
A resilient microbiome is one that is able to return to its original state after short-term disturbances such as antibiotic treatment, infections, or dietary changes. This plasticity ensures continuity in vital functions and helps prevent the overgrowth of pathogenic species.
Microbiome metabolites are small molecules produced by the microorganisms that reside in various parts of the human body, such as the gut, skin, and oral cavity. These metabolites play crucial roles in maintaining health and influencing various physiological processes. Products such as SCFAs, bile acids (BAs) and tryptophan metabolites are frequently referred to when assessing gut health and gut microbiota composition.
- SCFAs production by prebiotics has a pivotal role in maintaining gut barrier integrity, modulating immune responses and serving as an energy source for colonic cells.
- BAs, synthesized from cholesterol in the liver and subsequently modified by gut bacteria (i.e. deconjugation from glycine or taurine, dihydroxylation, oxidation or reduction, or epimerization), are also valuable indicators of microbial influence on host metabolism and gut integrity.
- Microbial fermentation in the gut results in the production of gases. The production of gases such as hydrogen, methane and hydrogen sulfide is another significant indicator of gut health and optimal microbial composition.
The Promise of Personalized Microbiome Medicine
Each person’s microbiome is as unique as their DNA. By understanding its variability, we can:
- Personalize diets and probiotics
- Develop precision therapeutics (e.g. postbiotics)
- Predict disease risk based on microbial signatures
Ongoing research explores microbiome interventions in infectious diseases, inflammation and immunology, neurodegenerative disorders, and oncology, and numerous biopharmaceutical companies are leveraging the therapeutic potential of the gut microbiome in various disease areas:
- Infectious diseases: Freya Biosciences, Infant Bacterial Therapeutics
- Immunology & inflammation: Vedanta Biosciences, Seres Therapeutics, Servatus Biopharmaceuticals
- Neurodegenerative disorders: LISCure Biosciences, Axial Biotherapeutics
- Oncology: MaaT Pharma, Microbiotica, Exeliom Biosciences
These companies highlight how microbiome-based interventions are moving toward clinical relevance in areas of high unmet medical need.
Examples
Among recent innovations, Perseus Biomix has introduced DynaMAP (Dynamic Microbiome Abundance Profiling), an amplification-free tool that enables highly sensitive and strain-level microbial profiling. It avoids PCR bias and allows researchers to track microbiome changes with unprecedented precision—ideal for probiotic impact studies and biomarker discovery.
Key Biomarkers of a Healthy Microbiome
|
Biomarker |
Role in Gut Health |
|
SCFAs |
Energy source for colon cells, anti-inflammatory effects |
|
Bile acids (BAs) |
Metabolic regulation, barrier function |
|
Gaseous products |
Indicators of microbial activity (H₂, CH₄, H₂S) |
|
pH balance (5.5–7) |
Supports beneficial species and enzymatic activity |
|
Inflammation markers |
Low CRP, calprotectin, and lactoferrin = reduced inflammation |
Tools like Breath Biopsy® (Owlstone Medical) allow non-invasive detection of volatile metabolites, reflecting microbiome activity.
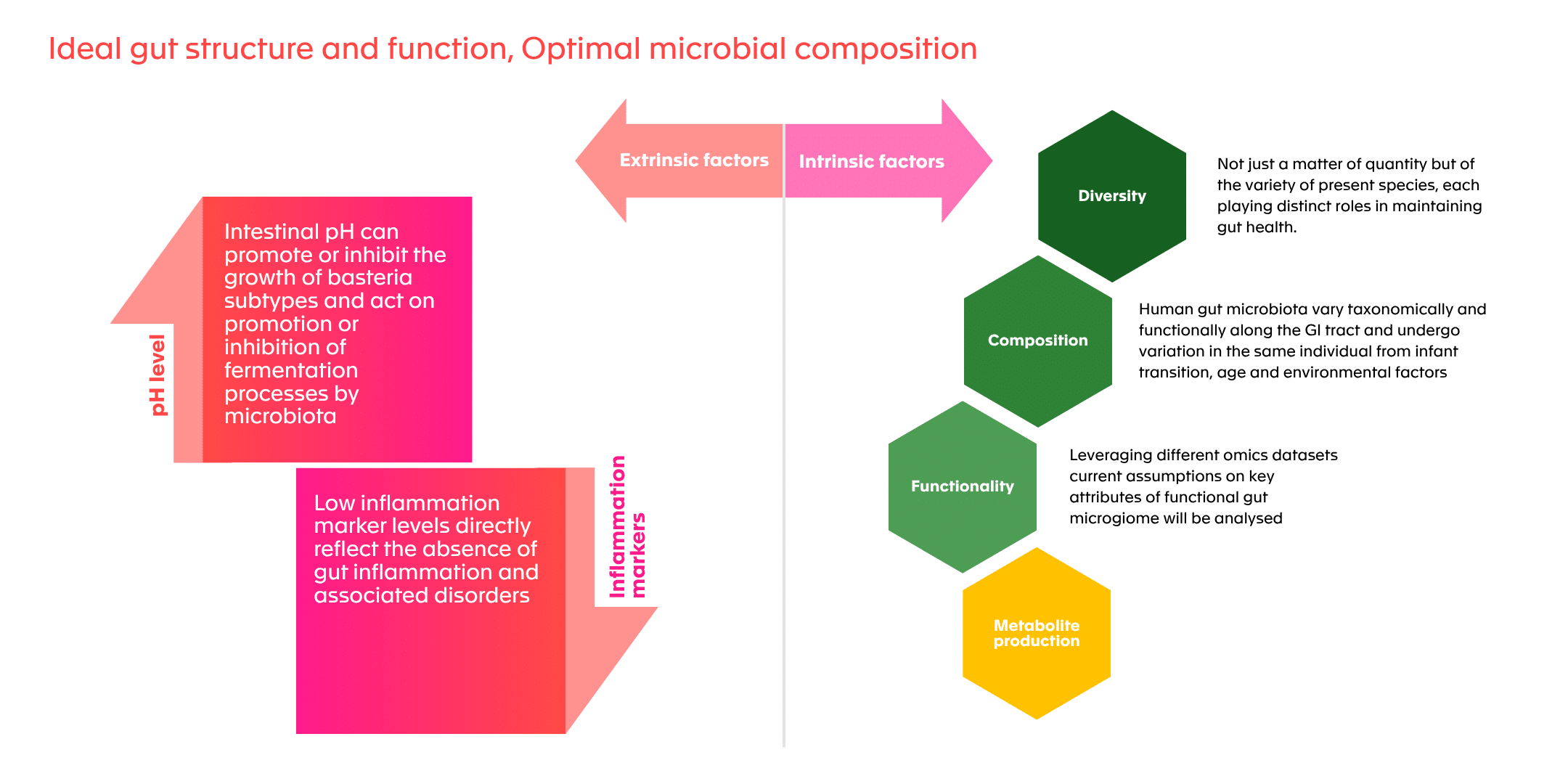
Ideal gut structure and function, optimal microbial composition
The following figure summarizes the principal indicators used to assess gut microbiota health: metabolite production (SCFAs, BAs), pH, inflammation markers, and gas production. It helps identify the multi-dimensional nature of microbiome evaluation.
Figure 2: Factors identified as healthy gut markers (modified from (Van Hul et al., 2024)**)
Technology Landscape: Tools for Microbiome Research
Multi-Omics: The Key to Understanding Microbiome Complexity
Modern microbiome science increasingly relies on integrated multi-omics approaches to understand how microbes influence health and disease. These include:
- Metagenomics: what is there
- Metatranscriptomics: what they express
- Metaproteomics: which proteins are produced
- Metabolomics: what metabolites result from microbial activity
Combining these layers allows researchers to move from microbial presence to microbial function and ultimately to clinical impact.
The table below highlights the main pros and cons of each technique.
|
Technology |
Pros |
Cons |
|
16S rRNA sequencing |
|
|
|
Metagenomics |
|
|
|
Metatranscriptomics |
|
|
|
Metaproteomics |
|
|
|
Metabolomics |
|
|
Table 1. Technologies used for microbiome studies
Roadblocks to Clinical Implementation
Several barriers must be addressed:
- Variability in sample handling, processing and analysis
- Artifacts and biases induced by contamination and annotation errors
- Lack of inter-study reproducibility
Solutions:
Manufacturers should select methods and materials based on specific research or diagnostic goals leveraging materials like NIBSC reagents for DNA extraction of gut microbiome samples and for NGS analysis. Standardized protocols for the complete workflow, from sample collection to processing, need to be implemented to minimize variability and bias. A rigorous validation of protocols should be established including calibration with known standards and inter-laboratory comparison. This is important as analyzing the microbiome is crucial for understanding gut health, disease mechanisms, and therapeutic interventions. However, several development challenges must be addressed to ensure accurate and reliable results.
Standardization Without Stagnation
While harmonizing protocols is critical for reproducibility and regulatory alignment, over-standardization can stifle innovation. Flexibility must be preserved to allow new technologies, methodologies, and microbial models to emerge. An adaptive framework—balancing standardization and research freedom—will be key to long-term progress in microbiome-based science.
Conclusion
While we may never define a single “healthy” microbiome, we can understand its functioning and learn to modulate it for better health. Precision tools and standardized methods will enable more accurate diagnostics, therapeutics, and patient-specific care pathways.
To support widespread clinical adoption, the field must invest in standardized, high-quality, and diverse reference datasets. These will enable benchmarking, regulatory alignment, and the future development of microbiome-based diagnostics and therapeutics.
As the field continues to evolve, regulatory and scientific guidance is essential for innovators developing microbiome-based diagnostics, therapeutics, and food products. A tailored development and regulatory approach is crucial.
References:
* World Health Organization. Constitution of the World Health Organization. 1946. https://apps.who.int/gb/bd/PDF/bd47/EN/constitution-en.pdf (accessed 10June2025).
** Van Hul M, Cani PD, Petitfils C, De Vos WM, Tilg H, El-Omar EM. What defines a healthy gut microbiome? Gut. 2024 Oct 7;73(11):1893-1908. https://doi.org/10.1136/gutjnl-2024-333378

Questions? Get the answer by our expert team
No two product developments are the same, talk to our experts about your development challenges and we will provide your actional recommendations.