The Nordic Opportunity: Turning Real-World Data into a Rare Disease Research Powerhouse

Over the past decade, rare diseases have gone from a niche to a major focus in pharma, thanks to regulatory incentives and advances in precision medicine. Biotech and big pharma have figured out that despite small patient populations, there are significant unmet medical needs, and these drugs provide sound business opportunities.
As of February 2025, over 20,000 clinical trials for rare diseases are underway globally, with nearly 4,500 in Europe alone.1 But focusing on rare and even ultra-rare diseases isn’t without its challenges. Recruiting participants for clinical trials is far from easy. You’re dealing with fewer eligible patients, and traditional randomized controlled trials (RCTs) may be either impractical or downright unethical. Enter real-world data (RWD). Its value has exploded.
Initially, RWD was used mainly to support market access and commercialization of new drugs. But now, it’s a must-have for things like natural history studies and external control arms (ECAs). And regulators get it. Both the FDA and EMA have published guidelines on how to use RWD in the regulatory processes.2,3 The FDA, as an example, supports complementing traditional evidence with RWD when there’s A) a significant unmet need, B) a rare cohort, C) a large expected effect size, or D) an existing body of evidence on a related population.2
Today, ECAs are essential for rare disease trials. They help to overcome some of the ethical and design challenges that come with small patient populations. They also have the potential to speed up clinical development, reduce the number of patients needed, and cut costs. And it’s not just talk anymore. Between 2019 and 2021, the FDA approved 88 drugs that used RWD to support safety or efficacy claims. Of those, RWD had a direct impact on the FDA’s decision-making in 65 (74%) cases, and RWD was included on product label in 38 (43%) cases.4
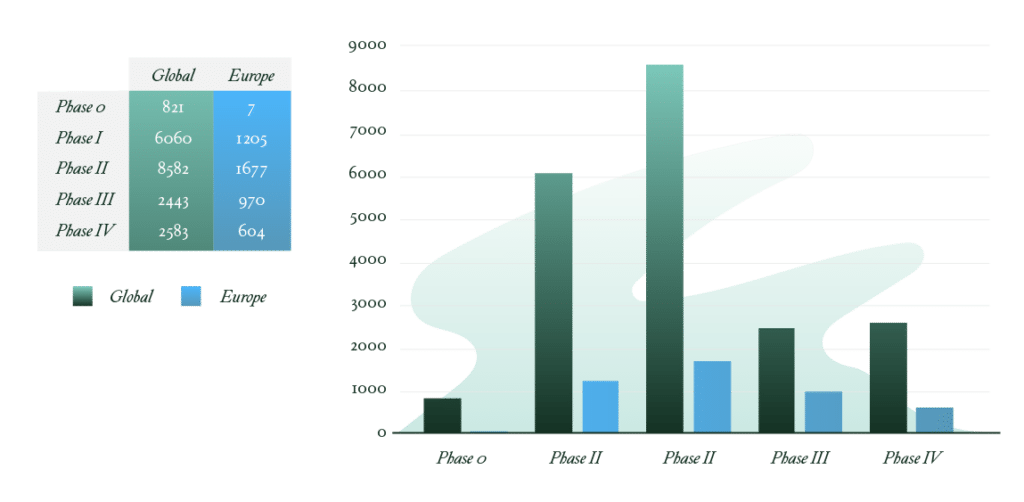
Number of Ongoing Clinical Trials in Rare Diseases
That said, building an ECA is no walk in the park. You’ve got to answer tough questions: What’s your source population? Can you pull high-quality RWD from the same population as your trial data? How do you harmonize variables between the trial and the ECA? How do you validate the outcomes? Which matching method is best? The list goes on.
The key question, though, is where to find the regulatory-grade RWD needed to build these ECAs. Here’s where the Nordic countries come in. With a combined population of 27 million, they could be the solution. Publicly funded healthcare systems, complete nationwide coverage, and robust digital national registers offer unique population-wide data. This eliminates some of the key biases seen in many other data sources. The Nordic countries also have modern data infrastructures, with individual-level data that spans multiple data sources and includes detailed clinical information. That kind of rich, longitudinal data is rare—and nearly impossible to replicate elsewhere.
Here’s the kicker: despite all of this, the Nordics have seen a steady decline in clinical trials over the years. That’s a problem. With the increase in clinical programs requiring regulatory-grade data, the Nordics have a billion-dollar opportunity on their hands. It’s time to turn the data advantage into a thriving clinical research ecosystem.
References:
1ⓒ GlobalData Plc. All Rights Reserved. Extracted Date: 27-Jan-2025;
3https://www.ema.europa.eu/en/homepage;
4Purpura et al. Clin Pharmacol Ther. 2022;111(1):135-144.
Blog post by MedEngine

Questions? Get the answer by our expert team
No two product developments are the same, talk to our experts about your development challenges and we will provide your actional recommendations.

